Difference between Boiling & Evaporation.
In common; both boiling and evaporation is the process in which liquid is getting converted into gas (vapor). Both the processes are direct function of temperature & pressure.
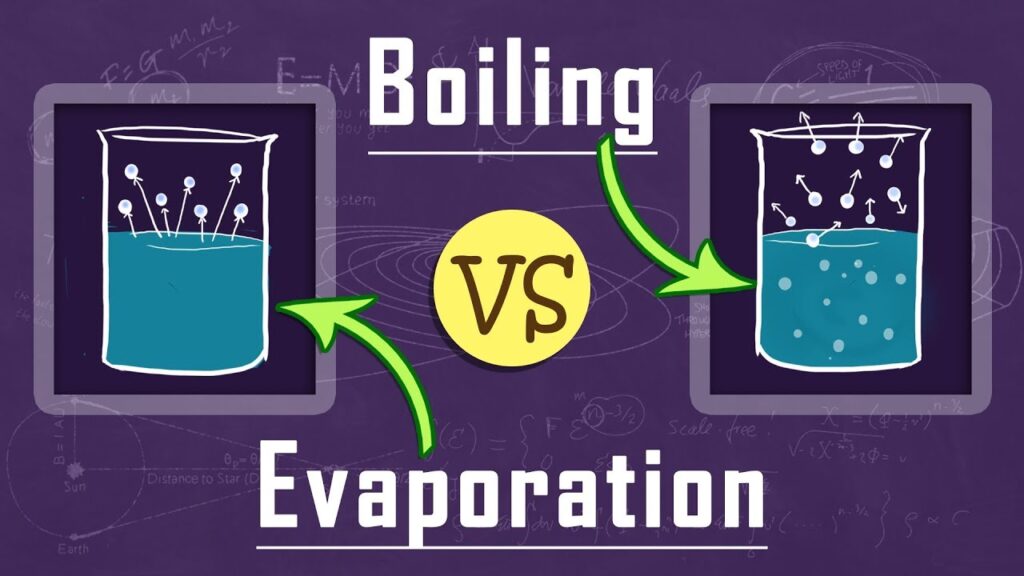
Boiling and evaporation is the process of a liquid becoming a vapor. This process is highly dependent on temperature. The higher the temperature of the liquid, the faster is the rate of evaporation. The maximum temperature at which (vapor pressure of the liquid is equal to the surrounding atmospheric pressure) a liquid changes into a vapor is called the boiling point.
In general boiling and evaporation is a highly confusing terms. Many times persons gets confused between both terms. Hence in this article let’s clear the difference between both of the terms.
Boiling :
- It’s a bulk phenomenon. It takes place throughout the liquid.
- Here the conversion of liquid to vapor takes place at the boiling point (at specific temperature and pressure).
- Its a rapid process.
- Boiling occurs when vapor pressure of the liquid is equal to the surrounding atmospheric pressure.
- Boiling is a dynamic equilibrium condition at which both; the rate of vaporization is equal to rate of condensation.
- Here bubbles of vapor forms in the liquid.
- During boiling liquid temperature remains constant throughout the liquid.
- Here in boiling rate of evaporation is independent of ambient Air RH (Relative Humidity).
Evaporation :
- It’s a surface phenomenon and doesn’t happen in a bulk.
- Here the conversion of liquid to vapor takes place below the boiling point. No specific temperature is needed for this, as it can occur on any temperature.
- Its a slow process.
- In case of evaporation as the vapor pressure increases evaporation occurs (at any temperature). Here the vapor pressure of the liquid is a fraction of the overhead pressure.
- Here no bubble forms / exists over the surface.
- Liquid temperature drops during evaporation.
- Rate of evaporation is a function of Ambient Air RH. As the RH of ambient air decreases evaporation decreases.
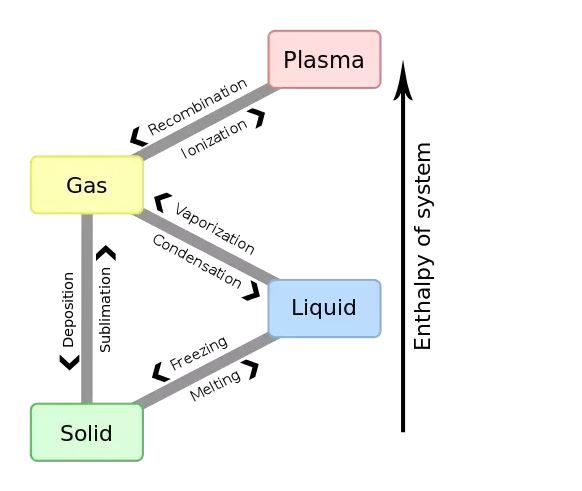
ImageSource : Wikipedia
Also Read:
Piping and Instrumentation Diagram – P&ID
How to choose betwwen PLC and DCS systems for process industries ?
Cement Manufacturing Process
Vinyl Chloride from Ethylene
Cooling Tower
Psychrometric Chart
What is Boiler ?
Venturi Flow Meter
Pitot Tube
Coriolis Mass Flow Meter
RECIPROCATING PUMP
Design of Centrifugal Pump
Valve & Its Types
Cavitation
P&ID Symbols and Notation
What is the Difference Between HMI and SCADA?
What is SCADA ? How does SCADA Works?
What is Programmable Logic Controller / PLC ?